The growing field of cannabis research is unlocking an entirely new world of medical, agricultural, and biochemical potential. From therapeutic applications to product formulation and quality control, cannabinoids standards play a vital role in ensuring accuracy, reproducibility, and global consistency in research data. Without standardized reference materials, the identification and quantification of cannabinoids would vary between laboratories, leading to unreliable results and misinterpretation of findings.
This blog explores the importance of cannabinoids standards, their scientific applications, and how they are driving innovation in cannabis research and analytical testing.
Understanding Cannabinoids and Their Diversity
Cannabinoids are naturally occurring compounds found in the Cannabis sativa plant. They interact with the body’s endocannabinoid system (ECS), influencing processes like mood, pain, appetite, and memory. The most well-known cannabinoids include tetrahydrocannabinol (THC) and cannabidiol (CBD), but over 100 different cannabinoids have been identified—each with distinct structures, biosynthetic origins, and pharmacological profiles.
Beyond THC and CBD, minor cannabinoids such as cannabigerol (CBG), cannabinol (CBN), and tetrahydrocannabivarin (THCV) are gaining research attention for their potential roles in inflammation control, neuroprotection, and metabolic regulation.
With this increasing chemical complexity, the need for cannabinoids standards becomes clear. Each compound must be correctly identified and measured, and that requires precise calibration tools—reference standards.
What Are Cannabinoids Standards?
Cannabinoids standards are high-purity reference materials used to calibrate analytical instruments such as gas chromatography (GC), liquid chromatography (LC), and mass spectrometry (MS) systems. These standards act as benchmarks for:
-
Quantitative analysis: Determining the concentration of each cannabinoid in a sample.
-
Qualitative analysis: Confirming the identity of cannabinoids through retention times and mass spectra.
-
Method validation: Ensuring analytical procedures meet accuracy and reproducibility requirements.
-
Quality control: Maintaining consistency across batches in research, product development, and regulatory testing.
When scientists use certified reference standards, they ensure that results obtained in one laboratory are comparable to those from another—an essential step for global cannabis science.
Why Cannabinoids Standards Are Essential in Research
1. Accuracy in Quantification
Inaccurate measurements of cannabinoids can lead to false claims in research and product labeling. Reference standards provide precise calibration for chromatographic and spectrometric instruments, minimizing analytical error. This ensures that reported THC or CBD concentrations truly reflect the sample’s content.
2. Reproducibility Across Laboratories
Different labs may use varying instruments, solvents, or methods. Standards serve as a unifying reference, allowing scientists around the world to generate reproducible data. This consistency supports scientific collaboration and regulatory compliance.
3. Quality Assurance and Regulatory Compliance
As medical cannabis and cannabinoid-based products become regulated, agencies such as the FDA, EMA, and Health Canada require validated methods supported by certified standards. Using cannabinoids standards demonstrates scientific rigor and adherence to regulatory expectations.
4. Advancement in Pharmacological Studies
Standards allow researchers to isolate and study individual cannabinoids’ pharmacological effects with precision. For example, understanding the subtle activity differences between THC and THCV requires accurate quantification of each molecule.
5. Product Development and Consumer Safety
In the cannabis industry, product formulation depends on reliable cannabinoid profiling. Whether designing therapeutic oils, edibles, or topical creams, standards ensure products are consistent, safe, and effective.
Analytical Techniques Using Cannabinoids Standards
Modern cannabis analysis combines chromatography and mass spectrometry technologies. Some common approaches include:
1. Gas Chromatography (GC)
GC separates volatile compounds, often after derivatization of acidic cannabinoids like THCA. Using reference standards helps calibrate detector response and identify peaks accurately.
2. Liquid Chromatography (LC)
LC, especially HPLC (High-Performance Liquid Chromatography), allows direct analysis of cannabinoids in their native acidic or neutral forms without derivatization. Calibration with standards provides precise quantification of both active and precursor forms.
3. Mass Spectrometry (MS)
Coupled with LC or GC, MS identifies cannabinoids by their mass-to-charge ratios. Reference standards are used to validate fragmentation patterns, ensuring compound identity and purity.
How Cannabinoids Standards Improve Data Integrity
Data integrity is a cornerstone of scientific credibility. Without accurate reference points, results lose meaning. Cannabinoids standards:
-
Enable instrument calibration for long-term accuracy
-
Facilitate interlaboratory comparison for reproducibility
-
Support method validation protocols required by ISO/IEC 17025 accreditation
-
Prevent false positives or negatives during cannabinoid profiling
-
Ensure traceability to certified suppliers and recognized metrological institutes
By minimizing technical bias and uncertainty, these standards help researchers focus on biological interpretation rather than analytical variability.
The Growing Market for Certified Cannabinoids Standards
As global legalization expands, the demand for certified reference materials is accelerating. Analytical labs, pharmaceutical companies, and universities require validated standards for both major and minor cannabinoids. Standardized libraries now include:
-
Δ9-Tetrahydrocannabinol (THC)
-
Cannabidiol (CBD)
-
Cannabigerol (CBG)
-
Cannabinol (CBN)
-
Cannabichromene (CBC)
-
Δ8-THC
-
Tetrahydrocannabivarin (THCV)
-
Cannabidiolic acid (CBDA)
-
Tetrahydrocannabinolic acid (THCA)
IROA Technologies contributes to this standardization effort by developing highly characterized reference materials, ensuring each compound’s identity, purity, and isotopic integrity are confirmed through advanced analytical techniques.
Challenges in Cannabinoid Standardization
Despite remarkable progress, developing high-quality standards comes with challenges:
-
Chemical Instability: Cannabinoids, especially acids like THCA and CBDA, can degrade or decarboxylate during storage or analysis.
-
Regulatory Complexity: Legal restrictions in some countries complicate transport and certification of cannabinoid reference materials.
-
Matrix Complexity: Cannabis extracts contain hundreds of co-eluting compounds that can interfere with detection.
-
Limited Access to Rare Cannabinoids: Minor cannabinoids occur in trace levels, requiring synthetic or biosynthetic production for standard creation.
IROA Technologies and other innovators are addressing these challenges through advanced synthesis, isotopic labeling, and high-resolution analytical verification.
IROA Technologies: Supporting Precision in Cannabis Research
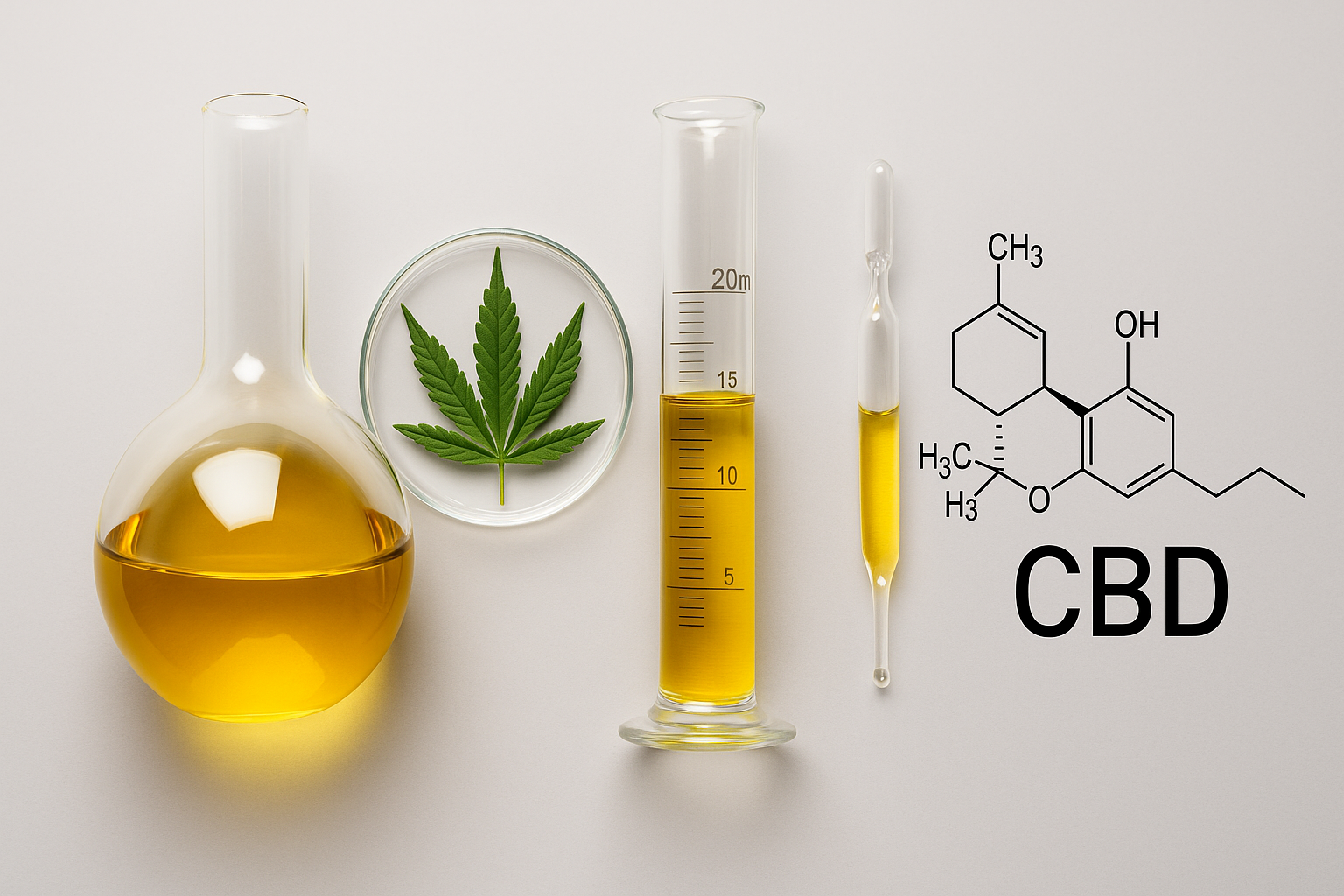 At IROA Technologies, we recognize that reliable metabolomic and cannabinoid data depend on validated standards and reproducible workflows. Our focus on isotopic labeling, internal standard sets, and metabolomic data analysis ensures that every measurement reflects biological accuracy rather than technical noise.
At IROA Technologies, we recognize that reliable metabolomic and cannabinoid data depend on validated standards and reproducible workflows. Our focus on isotopic labeling, internal standard sets, and metabolomic data analysis ensures that every measurement reflects biological accuracy rather than technical noise.
Through our cannabinoid and phytochemical standard libraries, we provide tools that enable scientists to explore cannabis metabolism, product quality, and therapeutic potential with confidence.
Learn more about reference standards and data reliability in metabolomics from the National Institute of Standards and Technology (NIST).
FAQs
1. What are cannabinoids standards used for?
Cannabinoids standards are reference materials used for calibrating instruments, validating analytical methods, and ensuring accurate quantification of cannabinoids in research and product testing.
2. Why are cannabinoids standards important in cannabis research?
They ensure consistent and reliable data across laboratories, enabling global comparison of results and compliance with regulatory frameworks.
3. How do cannabinoids standards improve product safety?
By verifying the concentration and purity of cannabinoids in formulations, standards help ensure consumer products are labeled accurately and meet safety requirements.
4. Can cannabinoids standards be used for minor cannabinoids like CBG or THCV?
Yes. Certified standards exist for both major and minor cannabinoids, allowing researchers to quantify even low-abundance compounds precisely.
5. Where can I find certified cannabinoids standards?
Certified cannabinoids standards can be sourced from specialized analytical suppliers and scientific organizations focused on metabolomic and phytochemical reference materials, such as IROA Technologies.
Conclusion
The role of cannabinoids standards in advancing cannabis research cannot be overstated. From ensuring data reliability and analytical precision to facilitating regulatory compliance and product development, these standards form the foundation of modern cannabis science. As the global cannabis industry continues to evolve, standardized analytical practices will become essential not only for scientific progress but also for consumer safety and product transparency.
By supporting the adoption of certified cannabinoid standards, organizations like IROA Technologies are helping shape a future where cannabis research is defined by accuracy, reproducibility, and scientific integrity.







