Metabolomics is a powerful tool for understanding the complex biochemical processes that define life. It enables researchers to study small molecules and their interactions within biological systems, offering insights into disease mechanisms, drug efficacy, and metabolic health. However, the accuracy of metabolomics data depends heavily on one critical factor Internal Standard Sets.
Without proper standardization, even the most advanced mass spectrometry or chromatography instruments can produce data that’s inconsistent, incomparable, or misleading. This is where Internal Standard Sets become indispensable, ensuring that every dataset reflects true biological changes rather than technical variability.
What Are Internal Standard Sets?
Internal Standard Sets are reference compounds added to biological samples before extraction or analysis. These standards, often isotopically labeled (like ¹³C or ¹⁵N compounds), serve as benchmarks to normalize and calibrate results throughout the workflow.
Their primary role is to compensate for technical differences in sample preparation, extraction efficiency, injection volume, and ionization behavior in mass spectrometry. By using stable isotope–labeled analogs of target metabolites, researchers can directly compare the signal of a standard with its corresponding endogenous metabolite.
In simple terms, internal standards ensure that what you measure truly reflects the biology—not experimental noise.
Why Metabolomic Analysis Needs Internal Standard Sets
Metabolomic data is highly sensitive to minute variations in temperature, solvent purity, instrument performance, and sample handling. Without correction factors, these variations can skew results and make it nearly impossible to compare data across samples, labs, or time points.
Here’s how Internal Standard Sets improve reliability and reproducibility:
1. Correcting Technical Variability
Each step of the metabolomics workflow—from extraction to injection—introduces potential bias. Internal standards account for losses during sample preparation and variability in detector response, ensuring accurate quantification.
2. Enhancing Data Normalization
Even when using high-resolution mass spectrometers, signal intensity can fluctuate between runs. Internal Standard Sets normalize these variations, allowing true biological differences to stand out clearly in comparative studies.
3. Improving Quantitative Accuracy
Absolute quantification is essential for biomarker validation, clinical metabolomics, and drug metabolism studies. Using isotopically labeled standards enables precise concentration determination of metabolites.
4. Ensuring Cross-Platform Comparability
Different analytical instruments or software platforms may process data differently. With Internal Standard Sets in place, researchers can confidently compare results between LC-MS, GC-MS, or NMR analyses.
5. Supporting Regulatory Compliance
In pharmaceutical and clinical applications, reproducibility is not optional. Internal Standard Sets help meet strict analytical validation requirements set by regulatory bodies such as the FDA and EMA.
How IROA Technologies Enhances Standardization in Metabolomics
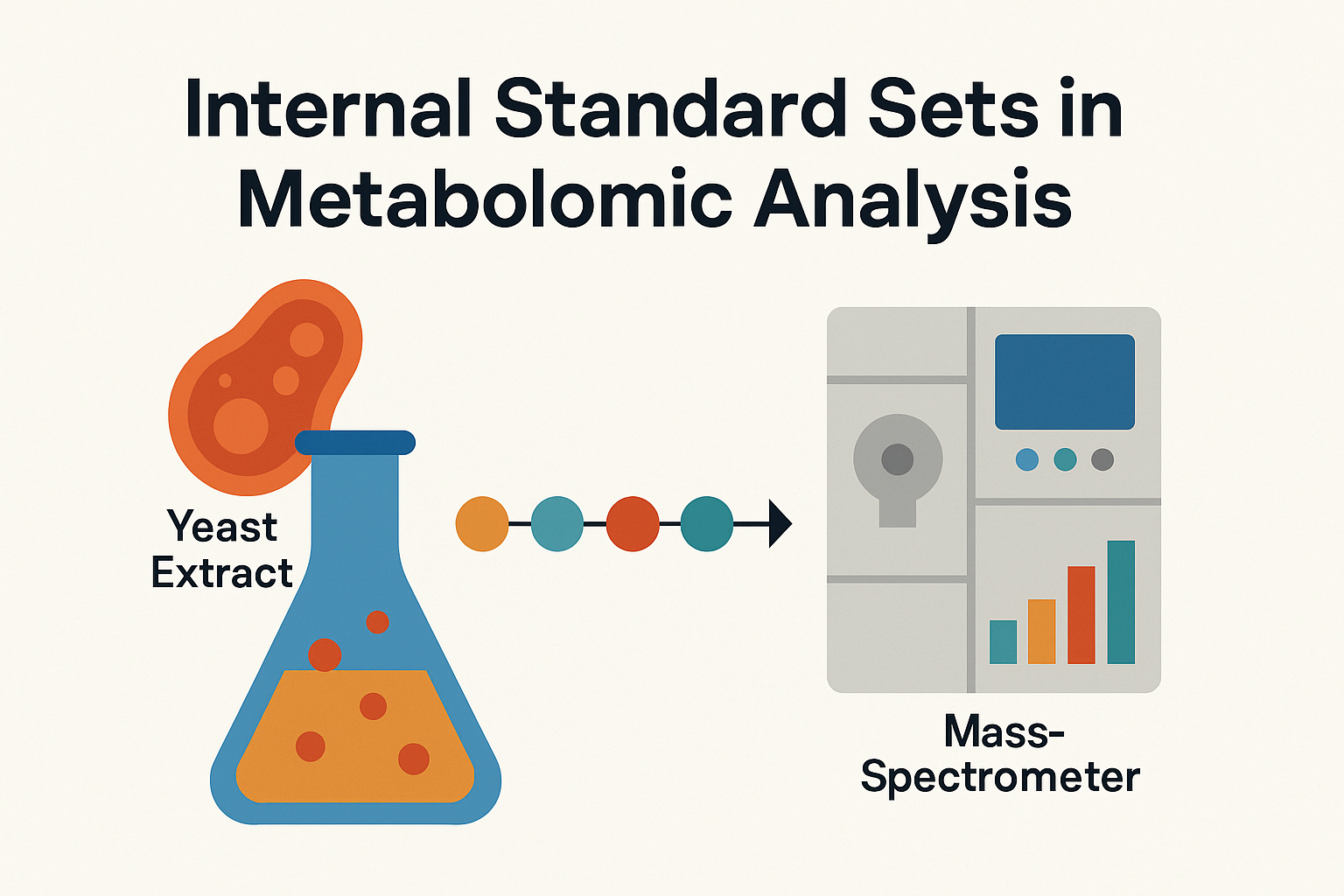 At IROA Technologies, we understand that metabolomic accuracy begins long before data analysis—it starts with reliable reference materials. Our Internal Standard Sets are designed using U-¹³C labeled metabolite yeast extracts and isotopically enriched compounds that mirror biological diversity.
At IROA Technologies, we understand that metabolomic accuracy begins long before data analysis—it starts with reliable reference materials. Our Internal Standard Sets are designed using U-¹³C labeled metabolite yeast extracts and isotopically enriched compounds that mirror biological diversity.
IROA’s unique Isotope Ratio Outlier Analysis (IROA®) methodology leverages stable isotope labeling to eliminate analytical variation. By applying both 5% and 95% ¹³C-labeled standards, researchers can accurately distinguish biological signals from noise, improving confidence in metabolite identification and quantification.
Our products are optimized for LC-MS and GC-MS platforms, offering consistent results across studies, instruments, and research teams. From complex biological matrices to targeted compound libraries, IROA’s solutions deliver unmatched reproducibility and scalability.
For more on the impact of isotopic labeling in metabolomics, you can visit the National Center for Biotechnology Information for related research.
Applications of Internal Standard Sets in Modern Research
The use of Internal Standard Sets extends across a range of metabolomics applications:
-
Clinical Research: Ensuring reliable biomarker discovery in diseases such as cancer, diabetes, and neurodegenerative disorders.
-
Pharmaceutical Development: Supporting drug efficacy and toxicity studies with reproducible quantitative data.
-
Agricultural and Environmental Studies: Quantifying metabolites in plant stress responses, soil health, or microbial ecosystems.
-
Food and Nutrition Science: Monitoring metabolic shifts related to diet, fermentation, and nutritional interventions.
By establishing robust baselines, Internal Standard Sets allow researchers to focus on biological interpretation rather than technical troubleshooting.
Common Mistakes When Not Using Internal Standard Sets
Even experienced researchers can underestimate the importance of standards. Here are some pitfalls of skipping them:
-
Inconsistent Data: Measurements may vary from day to day, making longitudinal studies unreliable.
-
Poor Reproducibility: Without correction, data cannot be replicated across labs or instruments.
-
Biological Misinterpretation: False positives or negatives may arise, leading to incorrect conclusions about metabolic pathways.
-
Wasted Resources: Repeating experiments due to poor standardization wastes time and increases costs.
In contrast, integrating Internal Standard Sets from the beginning of your workflow ensures analytical precision and saves both time and resources in the long run.
How to Choose the Right Internal Standard Set
Selecting the right standard set depends on your research focus, analytical platform, and target metabolites. Consider these factors:
-
Coverage: Choose standards that span a wide range of metabolite classes (amino acids, organic acids, fatty acids, etc.).
-
Labeling Type: Prefer stable isotope-labeled standards (e.g., ¹³C or ¹⁵N) for direct comparability.
-
Matrix Compatibility: Ensure that the standards are compatible with your sample type—plasma, urine, tissue, or microbial cultures.
-
Vendor Reliability: Opt for suppliers with proven expertise in metabolomics, such as IROA Technologies, where standards are designed by scientists for scientists.
FAQs
1. What are Internal Standard Sets in metabolomics?
Internal Standard Sets are reference compounds—often stable isotope-labeled—that correct for variability during sample preparation and analysis, ensuring accurate quantification of metabolites.
2. Why are Internal Standard Sets important?
They reduce technical variation, improve reproducibility, and make results comparable across instruments, time points, and laboratories.
3. Can I use a single internal standard for all metabolites?
No. Since metabolites vary widely in structure and behavior, multiple standards representing different compound classes provide better coverage and accuracy.
4. How do I choose the right Internal Standard Set?
Select a set that matches your target metabolites and analytical platform. Vendors like IROA Technologies offer tailored solutions for LC-MS and GC-MS workflows.
5. Are Internal Standard Sets required for clinical validation?
Yes. Regulatory guidelines for clinical and pharmaceutical studies mandate the use of internal standards to ensure reproducible, traceable results.
Conclusion
Reliable metabolomic data begins with reliable standards. Internal Standard Sets are the foundation of quantitative accuracy, helping researchers separate real biological signals from experimental noise. From normalization and quantification to cross-study reproducibility, their role is indispensable in modern analytical workflows.
At IROA Technologies, we provide scientifically validated internal standards and isotopic reference materials that support your journey from raw data to meaningful insights. With our commitment to precision and innovation, we make metabolomics more reliable, reproducible, and ready for real-world impact.